Abstract
Allogeneic stem-cell transplantation is an important curative strategy in acute myeloid leukaemia (AML) consequent upon the development of a graft-versus-leukaemia (GVL) effect. Disease relapse is the major cause of transplant failure and novel therapeutic strategies are required in patients with measurable residual disease (MRD) post-transplant. Recognizing that full donor T cell chimerism serves as a biomarker of GVL and that mixed donor T cell chimerism reflects the presence of bi-directional tolerance, we report the first prospective correlation of the impact of post-transplant T cell chimerism and MRD in patients allografted for AML/MDS.
Methods: 186 patients from FIGARO, a prospective randomized trial of reduced intensity conditioning regimens in AML/MDS, were alive and relapse free, at the first MRD timepoint and provided post-transplant bone marrow samples for flow cytometric MRD monitoring (day+42, month+3/+6/+9/+12) and peripheral blood samples for T cell chimerism analysis (3-monthly during the first year). Results were not available to clinicians. Flow cytometric MRD incorporating an unsupervised immunophenotypic analysis approach was used on 778 sequential samples. The prognostic significance of post-transplant MRD from each sampling timepoint was assessed by landmark analysis.
Results: 29 (15.6%) patients were MRD+ at one or more timepoints in the first year after transplant. The presence of post-transplant MRD was associated with reduced OS (HR:2.19, p=0.0026) and RFS (HR:5.36, p<0.0001), when analyzed as a time varying Cox variable. We, and others, have shown that pre-transplant MRD status is an important determinant of post-transplant survival. We therefore performed a multivariate analysis of factors that may determine the prognoses of patients with pre-transplant MRD+ disease. This demonstrated that the presence of detectable post-transplant MRD was the only independent determinant of RFS in this group of patients (HR 9.05 (95%CI:1.70-48.2), p=0.0099), even when adjusted for cytogenetics, FLT3-ITD and GVHD. The highest frequency of post-transplant MRD positivity occurred at day+42/month+3. The 2yr OS of day+42 MRD+ patients (n=13/158) was 30.8% (95%CI 9.5-55.4) compared to 67.1% (95%CI 58.5-74.2) in MRD negative patients (p<0.001); and 2yr CIR in day+42 MRD+ patients was 92.3% (95% CI:35.8-99.4) versus 22.8%% (95%CI:16.3-29.9) when MRD negative (p<0.001). Day+42 MRD was associated with more rapid relapse kinetics (median RFS of 2 months) compared to when MRD was first detected at later timepoints.
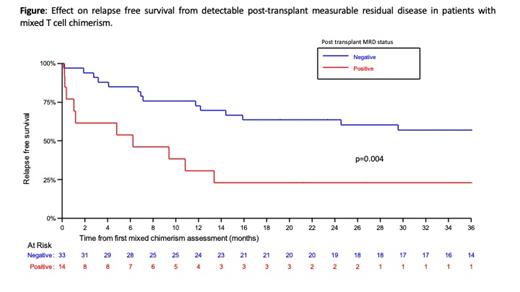
Next, we examined the impact of T-cell chimerism, and post-transplant MRD on outcomes. In patients with mixed-donor T-cell chimerism (MDTC) at month+3 or +6, the presence of post-transplant MRD (preceding or at time of first MDTC) was associated with decreased OS (p=0.001) and RFS. Specifically, 2yr RFS in MRD+ patients was 23.1% (95%CI:5.6-47.5), compared to 63.6% (95%CI:44.9-77.5) in patients who were MRD negative (p=0.004) (Figure). In contrast, in 48 patients with full donor T-cell chimerism (FDTC) at months 3 and 6, only 5 patients were MRD+ post-transplant and their outcomes were equivalent to patients who were MRD negative (FDTC/MRD negative 2yr RFS: 80.9% (95% CI:65.3-90.0)).
Downregulation of HLA class II molecules on AML blasts after transplant may result in immune evasion from GVL: we examined whether this had an additional impact on outcome in post-transplant MRD+ patients. Both RFS and OS were significantly lower in MRD+ patients when MRD+ blasts had down-regulated HLA-DR expression. In MRD+ patients, 2yr OS was 20.0% (95%CI:3.1-47.5) in those with HLA-DR down-regulation, versus 57.9% (95%CI 33.2-76.3) in those without (p=0.001).
Conclusion We demonstrate that dynamic assessment of post-transplant MRD is an important predictor of outcome in patients allografted for AML/MDS, with additional prognostic value to pre-transplant MRD assessment. Post-transplant MRD and T-cell chimerism results are most informative when combined, underlining the importance of a GVL effect in AML/MDS. This suggests post-transplant strategies which result in FDTC may improve outcomes in patients allografted for AML/MDS. Our data also supports the clinical relevance of monitoring for leukemic immune escape as HLA-DR down-regulation identifies a post-transplant MRD positive subgroup at risk of almost inevitable relapse.
Loke: Novartis: Other: Travel; Janssen: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Daichi Sankyo: Other: Travel. Protheroe: Jazz Pharmaceuticals: Honoraria; Astellas: Honoraria; Kite Gilead: Honoraria. Dillon: Amgen: Other: Research support (paid to institution); Astellas: Consultancy, Other: Educational Events , Speakers Bureau; Jazz: Other: Education events; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: educational events; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Session chair (paid to institution), Speakers Bureau; Menarini: Membership on an entity's Board of Directors or advisory committees; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research Support, Educational Events. Craddock: Novartis Pharmaceuticals: Other: Advisory Board ; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal